试题详情
25℃时,用0.0500mol•L﹣1H2C2O4(二元弱酸)溶液滴定25.00mL0.1000mol•L﹣1NaOH溶液所得滴定曲线如图.下列说法正确的是( )
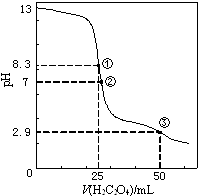
- A、点①所示溶液中:c(H+)+c(H2C2O4)+c(HC2O4﹣)=c(OH﹣)
- B、点②所示溶液中:c(HC2O4﹣)+2c(C2O42﹣)=c(Na+)+c(H+)
- C、点③所示溶液中:c(Na+)>c(HC2O4﹣)>c(H2C2O4)>c(C2O42﹣)
- D、滴定过程中可能出现:c(Na+)>c(C2O42﹣)=c(HC2O4﹣)>c(H+)>c(OH﹣)
知识点

参考答案
采纳过本试题的试卷