试题详情
25℃时,在含CH3COOH和CH3COOˉ的溶液中,CH3COOH和CH3COOˉ二者中各自所占的物质的量分数(α)随溶液pH变化的关系如图所示.下列说法不正确的是( )
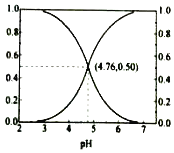
- A、在pH<4.76的溶液中,c(CH3COO﹣)<c(CH3COOH)
- B、在pH=7的溶液中,α(CH3COOH)=0,α(CH3COO﹣)=1.0
- C、在pH>4.76的溶液中,c(CH3COO﹣)与c(OH﹣)之和可大于c(H+)
- D、在pH=4.76的溶液中加盐酸,α(CH3COOH)与α(CH3COO﹣)之和保持不变
知识点

参考答案
采纳过本试题的试卷