选择题
下图是通过热化学循环在较低温度下由水或硫化氢分解制备氢气的反应系统原理,下列说法错误的是( )

- A、 反应②为反应③提供了原料
- B、 反应②也是SO2资源利用的方法之一
- C、 制得等量H2所需能量较少的是系统(I)
- D、 系统(I)制氢的热化学方程式为H2O(l) = H2(g) + 1/2O2(g) ΔH = +286 kJ/mol
在好氧菌和厌氧菌作用下,废液中NH4+能转化为N2(g)和H2O(l),示意图如下:
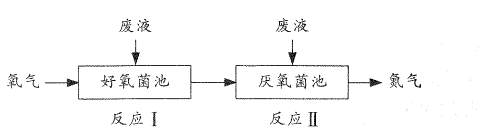
反应I
反应Ⅱ
下列说法正确的是( )
- A、 两池发生的反应中,氮元素只被氧化
- B、 两池中投放的废液体积相等时,NH4+能完全转化为N2
- C、 常温常压下,反应Ⅱ中生成22.4LN2转移的电子数为3.75×6 02×1023
- D、
联氨(N2H4)常温下为无色液体,可用作火箭燃料。下列说法错误的是( )
① 2O2(g)+N2(g)=N2O4(l) ΔH1
② N2(g)+2H2(g)=N2H4(l) ΔH2
③ O2(g)+2H2(g)=2H2O(g) ΔH3
④ 2 N2H4(l)+N2O4(l)=3N2(g)+4H2O(g) ΔH4=﹣1048.9 kJ·mol-1
- A、 O2(g)+2H2(g)=2H2O(l) ΔH5 , ΔH5>ΔH3
- B、 ΔH4﹦2ΔH3﹣2ΔH2﹣ΔH1
- C、 1 mol O2(g) 和2 mol H2(g)具有的总能量高于2 mol H2O(g)
- D、 联氨和N2O4作火箭推进剂的原因之一是反应放出大量的热
用CO通过下列方法可以合成甲醇。
①CO2(g)+2H2O(l) === CH3OH(g)+ O2(g) ΔH1=+764.5 kJ·mol-1
②CO(g)+ O2(g)===CO2(g) ΔH2=-283.0 kJ·mol-1
③H2(g)+ O2(g)===H2O(l) ΔH3=-285.8 kJ·mol-1
下列有关说法正确的是( )
- A、 反应①使用催化剂可以提高甲醇产率
- B、 反应②在任何条件下均能自发进行
- C、 反应③在100℃时ΔH<-285.8 kJ·mol-1
- D、 反应CO(g)+2H2(g)
 CH3OH(g)的ΔH=-90.1 kJ·mol-1
CH3OH(g)的ΔH=-90.1 kJ·mol-1
通过以下反应均可获取H2 . 下列有关说法正确的是( )
①太阳光催化分解水制氢:2H2O(l)═2H2(g)+O2(g)△H1=571.6kJ•mol﹣1
②焦炭与水反应制氢:C(s)+H2O(g)═CO(g)+H2(g)△H2=131.3kJ•mol﹣1
③甲烷与水反应制氢:CH4(g)+H2O(g)═CO(g)+3H2(g)△H3=206.1kJ•mol﹣1 .
- A、 反应①中电能转化为化学能
- B、 反应②为放热反应
- C、 反应③使用催化剂,△H3减小
- D、 反应CH4(g)═C(s)+2 H2(g)的△H=74.8kJ•mol﹣1
已知:2C(s)+O2(g)═2CO(g)△H=﹣217kJ•mol﹣1
C(s)+H2O(g)═CO(g)+H2(g)△H=bkJ•mol﹣1
H﹣H,O﹣H和O=O键的键能分别为436、462和495kJ•mol﹣1 , 则b为( )
- A、 +352
- B、 +132
- C、 ﹣120
- D、 ﹣330
Zn还原SiCl4的反应如下:下列说法正确的是( )
SiCl4(g)+2Zn(l)⇌Si(s)+2ZnCl2(g)△H1
SiCl4(g)+2Zn(g)⇌Si(s)+2ZnCl2(g)△H2 .
- A、 Zn(l)=Zn(g)△H=
(△H1﹣△H2 )
- B、 用硅制作的太阳能电池是将化学能转化为电能
- C、 增加Zn(g)的量,△H2变大
- D、 以Zn片、铜片和稀硫酸构成的原电池,Zn片表面有气泡产生.
 )是工业上合成各种塑料、离子交换树脂及合成橡胶的重要单体,工业上其制备原理是
)是工业上合成各种塑料、离子交换树脂及合成橡胶的重要单体,工业上其制备原理是 (g)
(g) (g)+H2(g)。请回答下列问题:
(g)+H2(g)。请回答下列问题: